The Central Institute for Experimental Medicine and Life Science (CIEM) is a global leader in the in vivo experimental medical arena. It is a very unique, independent organization that has never been controlled by a government organization, educational institution or a private company. This museum introduces the history of CIEM that extends to almost 70 years, together with various interesting events that have actually taken place over the decades.
| Age | Matters |
|---|---|
| 1947 | Tatsuji Nomura’s mother Masuko and sister Michiko started to rear mice as laboratory animals in their house. |
| 1951 | Kyoji Ando and Yoshio Tajima established the Experimental Animal Research Association. |
| 1952 | The Central Laboratory for Experimental Animals (CLEA) was established in Nishitama, Tokyo. Masuko and Michiko Nomura succeeded in establishing the Mongolian Gerbil as a laboratory animal. |
| 1954 | Supply of pelleted diet started. |

There are certain moments in life that could decide your destiny.
In the case of the current CIEA Chairman Tatsuji Nomura, that very moment came when he was having a
conversation with Dr. Koji Ando of the Tokyo University Epidemic Disease Research Institute. Dr. Ando
was his well revered boss at his workplace who continued to be his mentor in life for many years to
come. One day, Dr. Ando said to Tatsuji, “I admit that there is great meaning in continuing your
medical studies and making new discoveries through your research. But you could also go another way
and upgrade the level of the laboratory animals you are currently using. By doing so, you can raise
the entire level of medical research in Japan as a nation. So, which way will you go?”
That question was raised only five or six years after World War II when the completely battered
country of Japan was still trying to rise from the devastating aftermath of the war. Those were the
days when people had barely enough to eat. Consequently, laboratory animals were more like wild
animals that happened to be bred by farmers as a side job. Thus, quality was beyond question.
Against such a backdrop, Tatsuji Nomura gave up his medical research and became an experimental animal
breeder in 1954.
But Tatsuji actually had a good reason to become an animal breeder.
How and when did Tatsuji get acquainted with those animals for the first time?
Let us trace back to the very beginning of where everything started.

In 1945, Tatsuji graduated from the School of Science of Keio University.
His career was guaranteed at the Epidemic Disease Research Institute (current Institute of Medical
Science) of the University of Tokyo.
But due to some health problems, he was forced to take leave and rest at home for a while. While
recuperating, he thought of his work as a researcher.
As he dreamed on, he suddenly realized just how many laboratory animals he would need in his studies.
His dream was unrealistic considering the horrendously expensive price of the animals.
Those were the times just after World War II when there was a severe shortage of everything.
He also recognized that he was very unlikely to be given a chance of even getting close to those rare
laboratory animals.
After all, he was merely a young medical student fresh out of school and the most junior at the
workplace.
Agonizing over this dilemma, he eventually hit upon the idea of breeding those animals himself
while he was still resting at home in the seaside town of Oiso.
Hearing his idea, a close friend Ms. Hisako Mitsui, who later became Tatsuji's beloved wife, brought
him three pairs of mice. These mice were originaly brought by Hisako's father, Mr. Shigeo Mitsui from
Mitsui Chemical Industries. Tatsuji's mother, a great animal lover, took over the mice and started
breeding them at home.
This was how the Nomura Family started raising laboratory animals.

After some years, Tatsuji eventually went back to work for the Epidemic Disease Research Institute at Tokyo University, his original workplace.

There, he entered Dr. Koji Ando’s laboratory. Before and during World War II, Dr. Ando had worked at an upscale research institute in mainland China. Never having to worry about money, food or his work environment, Dr. Ando was taken aback by the dire poverty of his country upon returning. The inferior breeding conditions of the laboratory animals were especially overwhelming: Rabbits and mice died one after another, sitting in their many layers of excretions and bedding hay. Urged by a sense of need to do something about the situation, Dr. Ando consulted Dr. Yoshio Tajima, an old-time friend from his time in China.. Dr. Tajima happened to be working at the National Institute of Infectious Diseases located in the same building as Dr. Ando’s lab. He had a similar past as Dr. Ando, and had conducted studies in China receiving ample funding and breeding his own high-quality laboratory animals. He thus shared the same feelings and need as Dr. Ando, and the two doctors soon established the Experimental Animal Research Association in 1951.
Led by Dr. Ando and Dr. Tajima, the Experimental Animal Research Association preached the importance of improving the breeding environment of animals. It also stressed the need to provide high-quality laboratory animals to society. Such a stance and philosophy of the Experimental Animal Research Association was put into action by the Central Laboratory for Experimental Animals (CLEA), the private institution established by the Nomura family. Upon construction, Tatsuji's father Shunkichi Nomura provided the funding for the machinery. Meanwhile, Tatsuji's youngest brother Ryozo Matsukata prepared the necessary capital for the actual facility. Built within Ryozo’s farmland in 1952, CLEA took over the work of breeding mice that had so far been done by the Nomura family in Oiso. CLEA also started developing a pelleted diet and breeding containers to carry and deliver the reared animals to the clients.


The existence of Tatsuji's mother Masuko and his elder sister Michiko, should not be forgotten in the
history of CIEA. They were the two women who played a vital role in the early years of bringing up
CIEA’s laboratory animals.
While Japan was still rising from the ashes, Masuko and Michiko started breeding mice at their home,
once an elegant summer home of the Edo era ruler Tokugawa. Warming the breeding room with a briquette
stove when any heating means for humans were still lacking, they even turned their huge Western garden
into a vegetable patch for growing feed for the mice.

Bringing up the animals with utmost care, the ‘Nomura mice’ had become prime-quality laboratory
animals by the time they were sent to CLEA’s newly constructed facility in 1952. Masuko’s close
observation of animal behavior also became the precious base data for CLEA, upon its production and
supply start of a pelleted diet.
After all the mice at the Nomura home in Oiso had been moved to Ryozo’s farm, Masuko and Michiko
turned to other animals, assessing the possibility of establishing new laboratory animals. The
Mongolian Gerbil, which later became a popular experimental animal in the US and Europe, was one such
species they succeeded in establishing after seven years of trial and error.

| Age | Matters |
|---|---|
| 1956 | Contract commenced with the US Military 406 Medical General Laboratory to supply 60,000 mice and 12,000 kg of diet annually. |
| 1957 | The Central Laboratory for Experimental Animals was established as a nonprofit organization
under the direct control of the Ministry of Education. Contract with the US Military discontinued. Major salmonellosis outbreak occurred. |
| 1958 | Rearing facilities constructed in grounds of Tatsuji Nomura’s house for maintenance of mouse breeding stock. Aoyama branch opened. |
| 1959 | Meguro Branch opened.
Oizumi Branch (Gunma Prefecture) opened. All colonies in Nishitama were disposed of as elimination of salmonellosis was impossible. The Nishitama facilities were closed and demolished. Production of Wistar strain rats was discontinued because of a fire in rearing facilities in the Oizumi Branch. |
| 1960 | Grant received from the US National Institute of Health. |
| 1961 | First Specific Pathogen-free (SPF) animal facility completed in Meguro. |
| 1962 | SPF animal production facilities completed in Nogawa, Kawasaki.
SPF mouse breeding stock imported from the United States. SPF mouse production started in Nogawa. |
In 1953, the Silver Hamsters that the Nomura family had raised with utmost care all died at CLEA, due
to the inexperienced breeding skill of the laboratory staff.
This incident becomes the very moment in life for Tatsuji to decide whether he should continue being a
medical researcher or choose to become a laboratory animal breeder.
After continuously being preached the importance of raising the level of laboratory animals in Japan
by Dr. Ando, the boss of his workplace and leader in life, Tatsuji decided to become the latter in
1954.
The decision was not an easy one, though, and as Tatsuji started knocking on the doors of medical
schools, laboratories, pharmaceutical companies and so on, he found himself being looked down upon and
ordered to come through from the back door.
Even his former colleagues from school or work talked nastily about Tatsuji, saying that “he’s just
indulging in his pastime,” or “he’s given up research to make money by rearing those animals.” But
then, a helping hand was extended.
As Tatsuji continued his visits, he ran into the US Military 406 Medical General Laboratory.
The staff there appreciated the premium quality of the Guinea pigs that Tatsuji brought in, and rated
them as being “platinum class.” By 1955, the US facility therefore requested CLEA to supply 60,000
mice and 12 tons of diet on an annual basis.
This was a massive order reaching a total of 7.2 million yen.


In response to such a generous order from the US Military facility, construction work progressed at CLEA in Mizuho Town to expand its breeding buildings and improve its facilities. For the first time, the local bank also agreed to lend money without any mortgage. Backed by such tailwind, people around Tatsuji started suggesting that he should turn CLEA into a legally approved institution. Against such a backdrop, the Central Laboratory of Experimental Animals becomes a formal foundation on August 6th, 1957 by appointing Dr. Koji Ando as its Representative Director, and Tatsuji Nomura as its Director. It was a humble foundation however, with most of its paid-in capital of 5.99 million yen being in the form of the already used property and buildings borrowed from Tatsuji's father and brother. Meanwhile, the remaining minor monetary contributions were collected from Tatsuji’s friends.
Just as CLEA was institutionalized, the US Military abruptly terminated its contract. Two months
later in October, 1957, salmonellosis germs brought in from elsewhere quickly spread among the CLEA
mice. Consequently, the laboratory was forced to put down 6,000 mice in October, 1958. They then had
to euthanize another 5,000 in September, 1959. Even so, the breakout could not be stopped. Thus the
Mizuho Town facility was soon forced to be closed and demolished.
The saved handful of healthy breeding stock was evacuated to a small rearing hut hastily built
on the land where Tatsuji’s residence stood. Tatsuji considered this place as his last resort.
Concurrently, CLEA staff began searching for new land suitable for the production of large numbers of
laboratory mice.
By July, 1959, a new breeding facility was constructed in Oizumi Town of Gunma Prefecture. In order to
prevent rats from being infected with salmonellosis, they were also transferred to the new facility
together with the evacuated mice.
But then in December, another disaster struck. This time, the rat breeding building was burnt to ashes
by a fire.
Going through one disaster after another, CLEA was not in any condition to breed or sell laboratory animals on a regular basis. Worse, the existence of the laboratory itself was being questioned. Luckily, however, CLEA was rescued by the success of its pelleted diet. Ever since being institutionalized in 1957, the production and sales of CLEA’s original animal feed grew steadily, from a monthly supply of five tons in the beginning that came to exceed eight tons by 1960. Sales figures were also favorable for the pelleted diet, and while the sales profit of laboratory animals in 1957 was a mere 2.28 million yen, the profit for the pelleted diet reached 3.47 million yen. The sales for the pelleted diet exceeded that of the experimental animal sales, which was a trend that continued for almost a decade.


In 1958, CLEA’s Dr. Ando attended the First International Conference on Experimental Animals in
Paris.
There, he found out that the US and the UK were already joining hands to start full-fledged production
of SPF (Specific Pathogen Free) animals.
Determined not to be left behind by such a trend, CLEA also decided to establish and supply SPF
animals.
But to do so, there was a need to build a proper facility with strict sanitary and hygiene conditions.
This required a lot of money, which CLEA did not have.
Stuck in such a predicament, a helping hand was extended again from the US.
This time, help came from the NIH (National Institutes of Health).
NIH suggested to offer CLEA a total of 42.7 million yen assistance for four years, starting from 1959.
This encouraged CLEA to take further action, and in Japan, it negotiated with the Science and
Technology Agency to receive a total funding of 30.12 million yen up until 1962.
Furthermore, CLEA turned to the Japanese business community and was able to receive a total of 81.8
million yen of contributions by 1964.
In June, 1961, the first SPF facility was completed in Meguro.
Incorporating an eye-catching innovative cylindrical design, this two-storied concrete building
occupied a total floor space of 390 ㎡.
The disinfection and sterilization area was located to the right of the second floor, whereas the
clean room was to the left.
The clean room had a vinyl isolator for breeding germfree mice.
In order to maintain a completely aseptic condition, everything sent into the clean room was
disinfected or sterilized, including air, people, tools, water and feed.
After being grown for several weeks, baby mice in the clean room were transferred to the SPF vinyl
isolator.
There, they were colonized with specific microflara and became SPF mice.
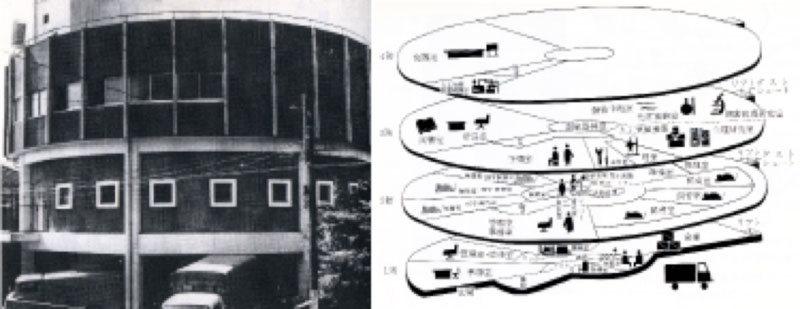

The SPF animal production facility in Nogawa was established in May, 1962.
This facility was to produce and breed SPF mice on a greater scale.
But things did not go as planned there. Upon birth, the weight of prenatal fetus is only around 1.5
grams.
Being so tiny, the staff had great difficulty in taking it out from the mother’s uterus by caesarean
section.
Without the breeding stock being germfree, no SPF mouse production system could possibly be
established.
Under such critical circumstances, CLEA issues an urgent order for 40 germfree mice to be imported
from the US.
In contrast, no such problems occurred for rats that are around 10 times larger than mice.
Hence their artificial nursing went smoothly, and CLEA had established their germfree breeding skill
by 1965.
| Age | Matters |
|---|---|
| 1963 | Supply of SPF mice started. Her Imperial Highness Princess Chichibu visited Nogawa Branch. Artificial nursing of rats achieved. |
| 1964 | Establishment of diet and environmental control and microbiologic testing methods for SPF
animals. Production of 20,000 SPF mice a month achieved. Supply of SPF rats started. Managing Director Kyoji Ando receives the Medal of Honor with Purple Ribbon from the Japanese Government. Monthly production of diet reaches 60 tons. |
| 1965 | Production/supply divisions separated to form the business entity, CLEA Japan Inc. Tatsuji Nomura is awarded the First Saburo Kojima Memorial Culture Prize. |
| 1965~1970 | Collaborative research with Armour Co. in the US. |
| 1966 | Reproduction and rearing of germfree animals achieved. Tatsuji Nomura becomes Director of CLEA. Medical Research Division established. Collaborative research with National Institute of Health (NIH) and Michigan University. |
R&D activities at CLEA started becoming very active from around 1963, when the production and sales of laboratory animals and pelleted diet finally went on track. Establishing the Medical Research Department in 1966, CLEA started to aggressively exploit the pre-clinical research area that contributes to the medical research and treatment of humankind.
The start of the full-fledged operation of the SPF animal production facility in Nogawa pushed up the
production number of laboratory animals and pelleted diet.
In figures, the supply number of mice reached 150,000 in 1963, 120,000 of rats, and 410 tons for
pelleted diet.
By 1964, the figures rose sharply to 250,000 for mice, 150,000 of rats and 543 tons for pelleted diet.
Consequently, the financial conditions of CLEA greatly improved, and the institution came to mark a
180 million yen profit by 1964.
Out of the total, 140 million was churned out by its business-operating divisions, while the
non-profit sectors marked a total profit of 36 million yen.
In total, the surplus reached almost 7 million yen.
Legally speaking, however, a foundation should be non-profit.
Hence, the business operating departments were separated from the activities of CLEA to form CLEA
Japan Inc. in February, 1965.


It is ironic that the strong growth of CLEA in the latter half of 1960s is owed greatly to the
Thalidomide Scandal.
Thalidomide was a sleeping tablet released by a West German pharmaceutical company in 1957.
To the shock of the international society, many children with malformed arms were born from mothers
who took Thalidomide in their early stages of pregnancy.
In Japan, 936 such children were born in the five years from 1958, when Thalidomide was first released
in Japan, until 1963, when its production was finally stopped.
This was the first time in medical history that humans found out that drugs did not only harm the
actual persons who took the dose but also those beyond, which in this case were the fetuses.
Hence in April, 1964, the Ministry of Health issued a notice of obligation to all pharmaceutical
producers, requesting them to attach a document of proof on having conducted appropriate animal tests,
whenever they applied for the approval and release of any new drug.
In response to this legal decision, all pharmaceutical companies in Japan scrambled to arrange their
own experimental animal facilities.
This followed with the request of a large volume of high-quality laboratory animals, and CLEA
consequently came to receive large orders from them.

A movement to consolidate a full-fledged research system arises, together with the progress of CLEA.
One move was to change the English name from the Central Laboratory for Experimental Animals (CLEA) to
the Central Institute for Experimental Animals (CIEA).
Another significant move was the establishment of the Medical Research Department in 1966 by inviting
Dr. Tomoji Yanagita, a post graduate of the University of Michigan who had specialized in the areas of
drug dependence and psychopharmacology, and was already world-renowned for developing a machine that
could measure an animal’s “quest for drugs.”
Dr. Yanagita actively promoted joint research with American universities, received consignment study
requests from overseas pharmaceutical companies, and conducted long-term joint studies with the World
Health Organization (WHO).
The Medical Research Department became more independent with the construction of the research facility
in Nogawa in 1968.
Its R&D activities progressed further from 1969 onwards in the five areas of reproductive physiology,
pharmacology, psychopharmacology, pathological toxicology and clinical pharmacology.
Despite such expansion in the research area, CIEA always focused its activities in the “preclinical
area,” thereby studying the cause and cure of human diseases by using laboratory animals.
To date, this basic principle of CIEA remains unchanged.
Approaching the Ford Foundation by selecting the study on monkeys as its main theme, CIEA succeeded
to receive a generous ten-year grant in 1967 that totaled to US $100,000.
In its second-term contract with the Ford Foundation that started from the autumn of 1972, CIEA
planned to turn Japanese Monkeys into laboratory animals by constructing their colony away from humans
and other animals.
Selected as the proposed location was an unmanned island called Edateku, far off the Amami Oshima
coast.
This plan had to be cancelled abruptly, however, as the island became subject for tourism development,
followed by another plan to build an oil refinery plant there.
Various voluntary research projects on small- to medium-sized monkeys were continued at CIEA even
after the contract with the Ford Foundation ended in 1977.
But costs of commodities and manpower were high in Japan, and production fees became astronomical.
Hence, CIEA’s action to turn monkeys into an experimental animal stagnated until artificial breeding
of crab-eating Macaques were commenced in 1988 on an unmanned island in Indonesia.
| Age | Matters |
|---|---|
| 1967 | Name changed from CLEA to CIEA (Central Institute for Experimental Animals). Koji Ando, Director Emeritus, was awarded the Order of the Sacred Treasure, Gold Rays with Neck Ribbon. Research grant from the US Ford Foundation commenced (US$100,000 over 10 years). |
| 1969 | International Council for Laboratory Animal Science (ICLAS) International Laboratory Animals
Asian Pacific Meeting Secretariat established. Gnotobiotes Research Facilities completed in Nogawa, Kawasaki. |
| 1970 | Japan EDM Co. Ltd. established. Collaborative research with Ciba-Geigy in Switzerland. Collaborative research with Schering Plough in the US. Collaborative research with the World Health Organization (WHO). |
| 1971 | Tatsuji Nomura becomes Japan’s Representative in ICLAS. |
| 1972 | Tatsuji Nomura becomes a member of the ICLAS Board of Directors. Tatsuji Nomura becomes Treasurer of ICLAS. Yoshio Tajima, Technical Consultant is awarded the Medal of Honor with Purple Ribbon. Muneo Saito, Technical Researcher is awarded the Mainichi Industrial Technology Prize. |
| 1973 | Biosciences Research Division established. |
| 1974 | Microbiology Building completed in Nogawa. Successful establishment of the rock rabbit as a laboratory animal. |
| 1975 | All divisions are brought together in Nogawa with the completion of research facilities. Leukocytosis phenomenon observed in nude mice for the first time in the world. Tatsuji Nomura receives the highest merit award of the Japan Medical Association. Nude mouse production reaches 10,000. |
| 1976 | The 2nd International Workshop on Nude Mice was held in Tokyo with Tatsuji Nomura as President. |
| 1977 | His Imperial Majesty Crown Prince Akihito (the current Heisei Emperor) visited the CIEA. |
| 1979 | The CIEA was designated as theICLAS Monitoring Center. |
| 1981 | The CIEA Support Group system started (34 companies). |
| 1982 | Developmental Engineering Research Laboratory established. |
| 1984 | Kosaburo Ezaki, Chief Investigator, received the Minister’s Prize of the Science and Technology
Agency. Tatsuji Nomura was awarded the Medal of Honor with Purple Ribbon. |
| 1985 | Laboratory Animal Monitoring Building completed. |
Based on its accumulated know-how over many years of research, CIEA started producing various kinds of germfree animals from the early 1970s. This, in turn, raised the issue of how the quality level of those animals could be maintained for a long time. That question eventually led to the establishment of the ICLAS (International Council for Laboratory Animal Science) Monitoring Center within the CIEA site in1986. By the end of the 1970s, CIEA started researching and developing physiological model animals with physiological functions and organs very similar to human beings. Furthermore, CIEA also actively studied disease-model animals suffering from similar sicknesses as human beings. Also sensational was the world’s first discovery of the G-CSF (Granulocyte Colony Stimulating Factor) by CIEA researchers in 1975. Inducing the number of white blood cells to soar at enormous speed, CIEA staff happened to come across G-CSF while they were studying the Nude Mouse in relation to human cancer.

Gnotobiotes are animals that possess clearly defined microbes.
They are very unique for discharging cancer-causing substances from the body in the original form,
even when injecting them directly into the intestines.
Having several other distinctive characters that contribute to medical research, gnotobiotes started
attracting much attention as a new type of experimental animal from the 1960s.
But in those days, Charles River, an American company, was the only place that could produce
gnotobiotes.
Furthermore, there were only a handful of institutions in the world that could supply germfree
breeding stock that would be necessary to produce those gnotobiotes.
As a consequence, all those animals were very expensive, and transportation costs were extremely high.
Hence, it was next to impossible for anybody in Japan to import and use gnotobiotes.
Based on the bitter past experience of failing to prepare germfree breeding stock mice and having to
import them from the US, CIEA staff had the strong desire to produce and supply a sizeable amount of
those animals.
Considering the situation at that time, CIEA chose to commit itself to the full-fledged development of
germfree mice, germfree rats and gnotobiotes in 1969.
By 1972, it established a monthly production system of 300 germfree mice, 700 gnotobiote mice and 200
gnotobiote rats.
At the same time, CIEA also established a consolidated system of gnotobiote inspection methods through
to their transport.
Such R&D activities were commended highly by the society, and CIEA comes to be awarded the Mainichi
Industrial Technology Prize in 1974.

Generally speaking, the genetic uniformity of an experimental animal is established by inbreeding a certain species for over 20 generations, or by forming and breeding a closed colony of a specific animal comprised of 20 to 30 individuals. But a problem rose in relation to such approach: While inbreeding raised genetic uniformity, it also heightened the natural occurrence rate of deformities. Moreover, it became relevant that controlled breeding of a closed colony sometimes reversed the distribution pattern of the blood type or raised the infection rate among the animals within the colony. To overcome such defects, CIEA started researching the establishment of hybrid animals in 1976. This was done by crossbreeding a certain group of uniform-quality animals with another group of uniform-quality animals. After much trial and error CIEA succeeded in the volume production of Multi-Cross Hybrid (MCH) mice and MCH rats by 1984, and started marketing them from CLEA Japan in 1985.

By the late 1970s, the need grew for the long-term quality control of laboratory animals after selling them to the clients. Hence in 1978, CIEA monitored the quality of laboratory animals by receiving funds from the Ministry of Education. This was the first time that such monitoring was ever conducted in Japan. Specifically, CIEA scrutinized the issues of genetic transformation and microbes, together with the possibility of computerizing the monitored data. Around the same time, CIEA also suggested to the US National Institutes of Health (NIH) that the guaranteed quality of the inspected laboratory animals should be defined and shared on an international level. Accordingly, the International Council for Laboratory Animal Science (ICLAS) issued a notice in 1979 stating that a monitoring center conducting global standard checking should be established in each area around the world. In response, CIEA started to raise funds in 1981 to collect a total of 200 million yen for building such a facility in Japan. Meanwhile by 1986, CIEA established the world’s first ICLAS Monitoring Center within its laboratory grounds.
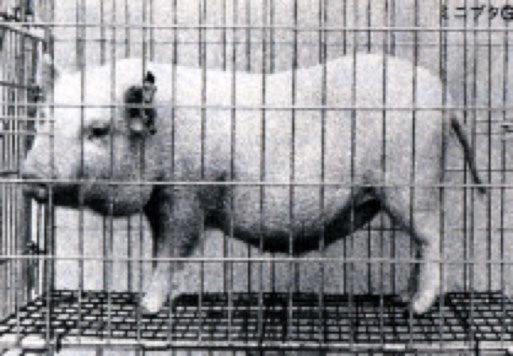
Physiological model animals can be used as human ‘models,’ since they have very similar physiological functions and organs as human beings. Furthermore, preparation of a wide phylogenic variety of such animals would be useful to conduct broad-scale safety tests of pharmaceuticals. According to such way of thinking, CIEA started the research and development of numerous animal species including the Gottingen Miniature Pig, Rock Rabbit, Volcano Rabbit and the Suncus Murinus.

Referring to their crossbreed and pathogenic status, as well as their ease of handling, CIEA started
trying to turn Gottingen Miniature Pigs into laboratory animals in 1975.
Weighing only 20 to 60 kilograms in contrast to ordinary pigs that weigh around 300 kilograms, the
project that CIEA started with eight female and two male Gottingens turned into a large-scale joint
research with Toray, receiving financial assistance from the Ministry of Education, as well as the
Ministry of International Trade and Industry.
By 1980, CIEA succeeded in turning the Gottingen Pigs into germfree animals, and formed a closed
colony of over 120 Gottingen Pigs with a uniform genetic character by 1982.
In 1984, CIEA led the world again by turning the Rock Rabbit into an experimental animal.
Weighing only around 300 grams and being much smaller than a common house rabbit which is around 4.5
kilograms, Rock Rabbits became a popular laboratory animal for being easy to handle, requiring smaller
breeding space and amount of feed, and thus being much cheaper and economical than a house rabbit.

CIEA also succeeded in the artificial breeding of the Volcano Rabbit, an endangered species, in 1985. By giving away a pair to the Jersey Foundation that works to protect and breed animals on the Red List, CIEA contributed to save the Volcano Rabbit that were once common animals found in the highlands of Mexico.

Meanwhile, CIEA also turned the Suncus Murinus of the mole family, which is unique for showing symptoms of seasickness, into a laboratory animal. Today, the Suncus Murinus has come to be used for studying the space sickness conditions of astronauts, as well as for scrutinizing the nausea symptoms caused by anticancer medicine.
The concept of using disease model animals to study specific diseases of human beings evolved from around the end of the 1960s. Corresponding to such thinking, CIEA has also developed a variety of such model animals.
Muscular dystrophy is caused by the lack of certain genes. When the disease progresses to a critical stage, the patient can die from respiratory failure. In search of the cause and an effective cure, the Ministry of Health established Japan’s first special research unit on muscular dystrophy in 1968. Becoming a member of this unit, Tatsuji Nomura was requested to expanded breeding and produce muscular dystrophy mice that are also found in the natural environment. By 1970, CIEA established a system capable of a monthly steady production of 50 muscular dystrophy mice. This was the first time that CIEA was involved in the production of a disease model animal. By 1972, CIEA started to work on producing SPF muscular dystrophy mice, and succeeded in doing so by 1983. Developing and producing other muscular dystrophy animals over the 20-year study period, the research unit eventually found out that the mechanism of muscular dystrophy caused in human beings is completely different from that of other animals.

AA Nude Mouse is a natural mutant with immune deficiencies.
Discovered for the first time in 1962, this mouse is short-lived, undergrown and has a low
reproduction capability as it has no thymus gland.
But due to the lack of this gland, it cannot eliminate intrusive foreign substances that invade from
outside, thus allowing easy transplantation of human cancer.
Taking note of the advantages the Nude Mouse offered in medical research, CIEA started the planned
mass production and breeding of Nude Mice from 1973.
By 1974, it established an annual production system of 300 such mice.
The annual production capacity of Nude Mice reached 15,000 by 1975, and a full production cycle
starting from the preparation of the breeding stock was established in 1976.
Concurrently, CIEA developed a highly sophisticated transplanting technology that contributes to human
cancer research.
This also enhanced the effectiveness of anticancer medicine measurement by using several hundred Nude
Mice at once.
Such results were obtained by CIEA staff, who systematically transplanted various different types of
human cancer on the mice, letting the cancer cells grow and colonize, removing the colonized cancer
cells and deep freezing them, and then transplanting the defrosted cancer cells onto other Nude Mice.
By repeating this process, CIEA has come to preserve over 400 different kinds of human cancer, a rare
and precious existence in the world.
While studying the Nude Mouse in 1975, CIEA researchers come across an abnormal incident where the white blood cell count suddenly increases. This was the first time that any person in the world observed the very moment when the human cancer cell induced the G-CSF to produce white blood cells. Since leucopenia could be effectively treated if this G-CSF could be specified, CIEA collaborated with Chugai Pharmaceutical in 1985 to promote further studies in this area. Finally in 2007, Chugai was able to release a medicine called Neutrogin, an effective treatment drug for leucopenia, based on CIEA’s original discovery in 1975.
| Age | Matters |
|---|---|
| 1986 | ICLAS Monitoring Center Building commenced operation. |
| 1987 | The Imperial Highnesses Prince and Princess Hitachi visited the CIEA. Reproductive Engineering Experimental Manual published. |
| 1988 | Tatsuji Nomura is awarded Muhlbock Memorial Prize at the ICLAS General Assembly Symposium. Special “Laboratory animal monitoring project” commenced. Hereditary disease clarification of the Shiverer Mouse successful for the first time in the world. |
| 1989 | “Life Programming I” and “Life Programming II” won prizes at the 30th Science and Technology Film Festival. Preclinical Medical Institute established. “Life Programming II” won prizes at the Italian International Film Festival. |
| 1990 | “Life Programming I” won the gold prize in the science department at John Muir Medical Film Festival in the United States |
| 1991 | Polio mouse created. “It Started with Six Mice,” a book depicting CIEA’s history, published. |
| 1992 | Laboratory animal cryopreservation facilities completed. |
| 1993 | WHO decided to use the polio mouse in its polio eradication program. |
| 1995 | RasH2 mouse was announced at the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH. Contributed to the shortening of the period required and the simplification of evaluation of results of carcinogenicity test for new drugs. |
| 2001 | US NPO Center for the Advancement of Health and Biosciences (CAHB) established. |
| 2002 | Overseas project development started. NOG mouse produced as “super immunodeficient mouse.” |
| 2005 | Patent granted for NOG mouse. |
| 2006 | Tatsuji Nomura becomes Director and Chairman of the CIEA Board. The 1st International Workshop on “Humanized Mice” was held in Tokyo with Tatsuji Nomura as President. |
| 2007 | Collaborative research on leukocytosis in nude mice with Chugai Pharmaceutical. Based on this phenomenon, lenograstim, a granulocyte colony-stimulating factor (brand name: Neutrogin) was marketed worldwide to treat leukopenia. |
| 2010 | World’s first closed colony of marmosets directly connected with regenerative treatment for spinal injuries and myocardial infarction maintained in the CLEA Japan Inc. |
| 2011 | Authorized as a public interest incorporated foundation New research facilities opened in Tonomachi, Kawasaki. |
A Shiverer Mouse cannot stop shivering because of its genetic abnormality.
In 1988, CIEA led the world by successfully tracing the cause of this disease.
This achievement accelerated CIEA’s R&D activities in the field of molecular biology, allowing its
researchers to develop and release a string of the world’s first genetically modified mouse.
To note a few of their achievements, the Polio Mouse contributed to evaluate the safety of live Polio
vaccines swiftly and accurately, the rasH2 Mouse established a new standard for the carcinogenicity
assessment of newly developed drugs, and the super immunodeficient NOG Mouse contributed to AIDS and
leukemia research.
More recently, Common marmosets that CIEA successfully turned into a laboratory animal are cultivating
new possibilities in the field of regenerative medicine, especially for treating spinal cord injuries
and cardiac infarction.
On the international level, CIEA is collaborating with other institutions to establish a global
standard for laboratory animals through the GALAS (Global Alliance for Laboratory Animal
Standardization).
As noted henceforth, CIEA has solidified its foothold as the world leader in laboratory animal preclinical research, by continuing to produce and provide high-quality, uniform-character laboratory animals to the medical arena for many decades.
And now towards the future, CIEA is renewing its determination to exploit a new horizon by preserving its basic stance of resolving the cause of various human diseases, developing better ways of medical treatment, and improving the health and welfare of all mankind.
◯Polio Mouse
From 1991 until around 1999, CIEA concentrated to develop the Polio Mouse. At the same time, it
worked to establish the safety evaluation of the live Polio vaccine, by using the Polio Mouse it
developed.
This was an exceptionally meaningful step on a global level, since live vaccines produced from the
attenuated Polio virus are used for immunization, and the safety of the vaccine must be carefully
checked to prevent it from accidental Polio caused by contamination of non-attenuated Poliovirus: A
disease that leaves behind a serious after-effect of paralyzing the extremities.
In the past, the above safety checking process was conducted by using monkeys. However, requests were
rising for a substitute laboratory animal, together with the growing demand in animal protection.
In 1978, Dr. Akio Nomoto of the Tokyo Metropolitan Institute of Medical Science succeeded in
establishing Japan’s first Polio Mouse. Approached by Dr. Nomoto, CIEA took over his research, and
turned the Polio Mouse into an experimental animal.

In 1993, the World Health Organization (WHO) conducted a comparative test on the Polio Mice and monkeys, to evaluate their efficacy on measuring the safety of the live Polio vaccine. This test confirmed the superiority of the Polio Mouse over a monkey, both in terms of test result accuracy and cost. The test also noted the significant meaning of substituting monkeys with mice, thus saving the precious mammal that delivered only one or two babies per pregnancy. Acknowledging the WHO’s test results, medical institutions around the world started replacing their monkeys with Polio Mouse whenever they checked the safety of their own live Polio vaccines.
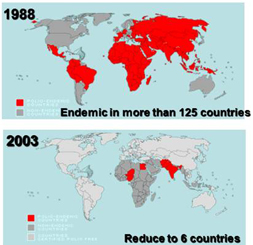
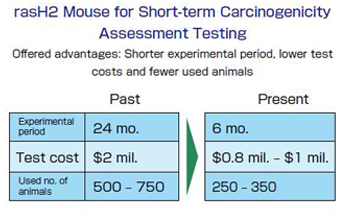
◯rasH2 mouse
The rasH2 Mouse is another mouse species that CIEA created in 1986, after about eight years of
trial and error.
Embedded with the ‘ras’ human cancer gene, this mouse was specifically developed for the
“carcinogenicity assessment test”: a compulsory examination process that is implemented during the
“safety evaluation test” of a newly developed drug.
In the past, this compulsory process was extremely unpopular for being expensive and time-consuming.
The dilemma was that it required an observation period of over two years after the chemical substance
in a new drug was continually administered to healthy mice and rats to check whether the substance
caused cancer or not.
Furthermore, even if that substance developed cancer in mice, it was very difficult to make a correct
judgment on whether or not it would also cause cancer among human beings.
Although the above shortcomings of the testing process were argued for many years, no effective
measures existed.
CIEA therefore created a big sensation in 1995 by announcing the rasH2 Mouse at the ICH (International
Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human
Use).
Cutting down the required time of carcinogenicity assessment tests from over two years to just six
months, the advent of the rasH2 Mouse even led the ICH to review its safety assessment process for new
drugs.
Following the rasH2 Mouse, other genetically modified mice, such as the p53 Mouse, the TgAC Mouse and
the XPA Mouse, were released in succession from various research laboratories in the US and Europe.
But a comparative test of the four different kinds of mouse conducted by the respective new drug
approving administrative organizations in Japan (Ministry of Health, Labor and Welfare), the US (Food
and Drug Administration: FDA) and the European Union (Committee for Proprietary Medicinal Products:
CPMP), evaluated the rasH2 Mouse most highly.
As such, the worldwide demand for the rasH2 Mouse in 2010 reached 23,000, turning it into the most
popular laboratory animal for carcinogenicity assessment even in the US that is controlled by the FDA.
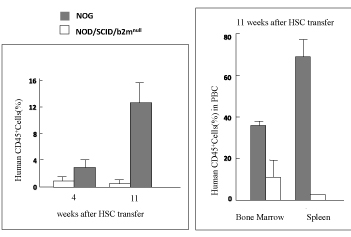
◯NOG Mouse
Almost fully eliminating the immune functions of a healthy mouse, the super immunodeficient NOG
Mouse was created for the first time in the world by the Mamoru Ito Research Team in 2002: another
remarkable achievement by CIEA.
In the natural environment, the Nude Mouse was the first immunodeficient mouse discovered in 1962.
The Nude Mouse suffered immunodeficiency as it had no thymus gland which produces T-cells.
This discovery was followed by another mouse with even more severe combined immunodeficiencies (SCID)
in 1983.
Named the SCID Mouse, this mouse had no T- and B-cells, a distinctive feature that allowed easy
transplantation of human lymphocyte cells.
Hence, the SCID Mouse soon became a popular experimental animal for studying HIV that infects and
grows in lymphocyte cells.
CIEA imported the SCID Mouse breeding stock in 1985 from the US, and tried to produce a mouse with no
immune function.
In doing so, the Ito Team discovered that a more immunodeficient mouse could be produced by crossing
over the SCID Mouse with a NOD (Non Obese Diabetic) Mouse.
But the SCID-NOD Mouse still retained a relatively large number of NK (Natural Killer) cells that are
responsible for some immune functions.
By the mid 1990s, the significant progress in the gene engineering technique allowed the normal immune functions of a healthy mouse to be artificially “knocked out.” Eventually, it also becomes possible to define which genes should be knocked out to reduce the immune functions to almost nil. Dr. Masataka Nakamura of the Tokyo Medical and Dental University used this technology to produce his NK cell knocked out genetically modified mouse. But his mouse still had a large number of T- and B-cells.
It seemed logical then for Dr. Nakamura, former member of Sugamura Group at Tohoku University who had
transferred himself to Tokyo Medical & Dental University,
to approach the Ito Team at CIEA and suggest that by collaborating with each other, the probability of
producing a more perfect immunodeficient mouse would increase greatly.
The outcome was the NOG Mouse, which is being praised globally today as the “ultimate form of an
immunodeficient mouse.”
Recording an astonishing 40% to 70% survival rate of human hematopoietic cells, the NOG Mouse
production by CIEA already reached 10,000 per year in 2006.
But in spite of such remarkable achievement, Mamoru Ito, the leader of the NOG Mouse Development Team,
humbly says “we had the luck and chance.”
Ito continued to state that “the laboratory animals we produce would be worthless if our clients don’t
use or value them.”
◯Marmosets
During the 1960s, CIEA already started studying monkeys, and tried breeding the South American
marmoset in 1973.
But having no idea on how they ate or lived, the 17 wild marmosets died within a month after being
brought over to Japan.
Though another 10 years were spent to turn it into an experimental animal, no demand rose for them for
a long time in Japan, since they were an uncommon monkey species among the Japanese.
Compared to other monkeys, however, marmosets are quite small like a rat and weigh only around 200 to
400 grams.
Furthermore, they offer the advantage of being much more fertile than macaque monkeys.
Having two to three babies per delivery after a six-month gestation, each mother can deliver around 50
babies in roughly 10 years.
As such, they started to be actively used in the regenerative medical field from around 2000.

Today, marmosets are most commonly used for studying spinal cord injuries and cardiac infarctions. As for the former, marmosets are used for developing the stem cell transplant method that recovers and reproduces paralyzed lower body functions caused by spinal cord injuries. As for the latter, research is conducted to ultimately reproduce the human cardiac muscle damaged by cardiac infarction, through new cardiac muscle cell transplantation using marmosets. As of 2010, CLEA Japan Inc., CIEA’s related company, maintained the world’s largest colony of marmosets comprising of 500 males and 500 females.
◯GALAS (Global Alliance for Laboratory Animal Standardization)
In order to standardize the quality of laboratory animals, GALAS was collaboratively established
by laboratory animal producing companies and institutions throughout the world,
according to the call from CIEA. In the autumn of 1998, CIEA joined hands with four experimental
animal producing companies in Japan, the US and Europe,
and signed a contract to regulate and standardize the quality of rats for laboratory use.
In the future, CIEA intends to expand the activities of GALAS to standardize the quality of many other
laboratory animals including marmosets.

Opening a new laboratory in the summer of 2011, in the coastal area of Kawasaki City across the newly re-internationalized Haneda Airport, CIEA will take a progressive step forward to nurture a new industrial foundation based on medical/new drug creation technologies, by collaborating with pharmaceutical companies, medical equipment-related industries, health/foodstuff-related corporations, venture businesses, and so on.
| Age | Matters |
|---|---|
| 2011 | The Central Institute for Experimental Animals (CIEA) certified as a public interest
incorporated foundation. New laboratories opened in Tonomachi, Kawasaki-ku, Kawasaki, Kanagawa. Designated as a Keihin Waterfront Area International Strategic Comprehensive Special Zone for Life Innovation. |
| 2012 | Japan Society for Marmoset Research established, and its Secretariat established at CIEA. Received “Conformity” rating by the Japan Health Science Foundation (HS) for external verification in animal care and use. |
| 2013 | Tatsuji Nomura, Research Director and Chairman, passed away. Ryuta Nomura was appointed as Chairman. Junichi Hata appointed as Research Director. “KING SKYFRONT Summer Science Event” started. |
| 2014 | Nomura Tatsuji Award established by the Keio Medical Society. |
| 2016 | “CIEA Science Camp” (Sponsored by CIEA. Subsidized by Children’s Dream Fund) started. The 63rd Annual Meeting of the Japanese Association for Laboratory Animal Science held; President: Mamoru Ito. |
| 2017 | A new Animal Live Imaging Center established. |
| 2020 | Awarded the 3rd Japan Medical Research and Development Awards in the Minister of State for Health and Medical Care Strategies Prize. Mamoru Ito appointed as Research Director. |
| 2021 | The rasH2 mouse is officially listed in the ICH S1B Guideline Addendum and recognized as a global standard system. |
| 2022 | Publication of “From Six Mice, 3 — How to Create a World Standard System: the CIEA Way” by
Ryuta Nomura. Celebrates its 70th anniversary on May 15. |
| 2023 | Makoto Suematsu appointed as Research Director. |
| 2024 | Name changed from CIEA to CIEM (Central Institute for Experimental Medicine and Life Science). |
CIEA was originally certified in 1957 as a foundation under the jurisdiction of the Ministry of Education; however, in accordance with the 2008 reform of the public interest incorporated foundation system, it was newly certified as a public interest incorporated foundation under the jurisdiction of the Prime Minister as of April 1, 2011, as a public interest organization that conducts “activities aimed at promoting academics and science technology.” In July of the same year, CIEA opened a new research establishment at the KING SKYFRONT, which was developed in the Tonomachi district of Kawasaki-ku, Kawasaki, and relocated entirely from Nogawa, Miyamae-ku, Kawasaki. This new research establishment is a state-of-the-art laboratory animal facility built based on a three-year concept, and we have embarked on a research and development (R&D) project to make further progress in this new location. Furthermore, in December 2011, the KING SKYFRONT area was designated as an “International Strategic Special Zone,” and will undergo further significant development.

In March 2011,the City of Kawasaki officially launched an urban redevelopment project to build the Tonomachi 3-chome area in Kawasaki-ku, the former site of Isuzu Motors’ Kawasaki plant, into a world-leading R&D center in the health and medical fields, naming the area “KING SKYFRONT.” To further promote this project, the three parties, including Kanagawa Prefecture and the City of Yokohama, applied for designation an “International Strategic Special Zone,” and on December 22, 2011, the project was approved as the “Keihin Waterfront Area International Strategic Comprehensive Special Zone for Life Innovation.” Currently, more than 70 institutions, including universities, research institutes, and companies, are located in this zone.
CIEA was the first to locate in this KING SKYFRONT area in response to the invitation by the Kawasaki City authorities, and has been developing its R&D business in its role as the core institution in this area.
CIEA has been expanding its R&D operations since 1974, when all of its departments were concentrated in Nogawa, Miyamae-ku, Kawasaki. However, in the 2000s, the entire facility began to become deteriorated, necessitating extensive renovation of the building and complete replacement of the attached machinery, including the air-conditioning system. Nevertheless, a solution could not be found in terms of funding. Under such circumstances, in 2008, Kawasaki City approached us about relocating to an urban redevelopment district in Tonomachi, Kawasaki-ku. Thankfully, this offer included the availability of a 1/3 subsidy program from the Ministry of Economy, Trade and Industry (METI) for the construction of the facility, which was made possible through the efforts of the Kawasaki City government. With this METI subsidy and the proceeds from the sale of the Nogawa land, the construction of the new research establishment became feasible, and the relocation plan took shape. In the same year, following the materialization of the plan, Chiyoda Techno Ace Corporation, which had been involved in the construction of the Nogawa facility, was selected to manage the construction, and discussions began with the construction committee of the new research establishment. The first issue discussed was the height restriction (25 m or less) due to the fact that the land leased from Kawasaki City was adjacent to Haneda Airport across the Tama River, and what kind of facilities could be built within that height limit. Details of the specific functions and machinery to be included in the new facility were finalized, and construction began in April 2010.
On the other hand, CIEA has completely reviewed its past research themes and projects, and clarified the subjects on which it should focus its efforts. The concept behind the construction of the new research establishment was as follows:
(1) Narrowing down the animal species to mice and common marmosets, we will develop these animals and
an animal experiment system using these.
(2) Separate mice, marmosets, and monitoring centers (MCs) by floor. This is because breeding methods
for both animal species are different and animals are brought to MCs from outside institutions for
testing. Furthermore, separate entrances and exits and animal elevators should be secured to avoid
crossbreeding on these three floors.
(3) The animal facility shall be an energy-efficient facility through the use of a new
air-conditioning system (Aqua Clean: Reduces ventilation frequency by reusing exhaust air from animal
facilities and by using module-specific airflow regulators (variable air volume (VAV) system)).
(4) Modularization of the animal and laboratory rooms (3.1 m × 6.2 m) will allow for alternate use
changes.
(5) Exterior corridors will be installed in animal facilities to enable easy control of temperature
and humidity in animal rooms, as well as to provide both noise insulation and condensation
prevention.
(6) Provide walkable ceilings to allow maintenance of facility equipment without access to the animal
rooms.
(7) Provide two types of emergency power sources, heavy oil power and gas turbine power generation,
for animal air-conditioning units, freezers, and so on.
(8) The working office shall be large rooms to promote mutual exchange of ideas among staff members.
As a result of these considerations, the new facility was designed to have a site area of 6,000 m2, a total facility area of 11,600 m2, and five floors. In addition, the number of personnel was initially assumed to be 100.
The new research establishment was completed in July 2011, three years after its conception, and a grand opening ceremony was held with concerned parties and local residents invited.
On January 11, 2013, Tatsuji Nomura, Research Director, and Chairman passed away at the age of 90. He served as director of CIEA since its founding in 1952, and also as its chairman since 2006. Throughout this period, he actively developed research and projects related to laboratory animals and animal experimentation, established the foundation of the concept of laboratory animals, and made significant contributions not only to laboratory animal science but also to other related research fields. His activities were not limited to Japan, but were global in scope, including serving as the Japanese representative to the US-Japan meetings on laboratory animal science and as an officer (Treasurer and Vice President) of the International Council for Laboratory Animal Science (ICLAS). He was awarded the Medal with Purple Ribbon in 1984, the Eiji Yoshikawa Cultural Prize in 1992, and designated a Person of Cultural Merit in 1997 in Japan. Overseas, he was also awarded the ICLAS Muehlenbeck Memorial Award in 1988, the U.S. Food and Drug Administration (FDA) Distinguished Alumni Award in 1998 and 2000, and the ICLAS Marie Coates Award in 2005.

“KING SKYFRONT Summer Science Event” (Executive Committee Chairperson: Ryuta Nomura) is a hands-on event started in 2013 in which universities, research institutes, and companies operating at the KING SKYFRONT make use of their own business activities to introduce science and technology to elementary school students in a casual way. CIEA has participated in this event since the beginning, and has received a lot of positive feedback from elementary school students after planning events to familiarize them with science and technology , and CIEA considers it an important opportunity to make contact with many people.
In 2014, a year after the passing of Research Director and Chairman Tatsuji Nomura, the Keio Medical
Society of his alma mater, Keio University School of Medicine, established the Nomura Tatsuji Award,
which is a great honor for us as CIEA. The president’s greeting on the Keio Medical Society expresses the
purpose of the award’s establishment as follows:
“(President’s Greetings) Dr. Tatsuji Nomura was a 24th year graduate of Keio University School of
Medicine. Dr. Nomura sadly passed away on January 11, 2013. As Research Director and Chairman of the
Central Institute for Experimental Animals, he devoted his entire life to the creation of
high-quality experimental animals, which are indispensable for the development of medicine, and to
the establishment of a comprehensive animal experiment system that enables the pursuit of plurality
standards and reproducibility. It is all too well known that the roots of his career can be traced
back to 1947, when his mother, Masuko Nomura, and her sister, Michiko, began raising mice at the
Nomura family residence in Oiso. Through a history of struggles to improve the bottom line of animal
experimentation science, Dr. Nomura has overcome hardships with his spirit of independence and
self-respect intact, and as a result, has created a world-class animal experimentation system. The
Keio Medical Society has established the ‘Tatsuji Nomura Award’ to honor Dr. Nomura’s achievements
in leading in vivo experimental medicine. Dr. Nomura was a researcher who always remained committed
to the development of animal experimental medicine with a clear clinical focus. I sincerely hope
that the creation of this award will be a catalyst for the further development of such medicine and
the development of novel medical research that will truly be of global value.” (Reproduced from
the Keio Medical Society website)
The camp has been hosted by CIEA since 2016 under the theme “Let’s learn about the process from the birth of life to the formation of the body.” This program aims to cultivate children’s understanding and interest in science through activities that familiarize them with science and science-related topics and is supported by the Children’s Dream Fund of the National Institution for Youth Education. The program is targeted at junior high school students, and the number of applicants always exceeds the limit, indicating the high level of need for the program. During the event, lectures are given on the birth of life and how the body is formed, while hands-on activities include DNA extraction experiments, observation of fertilized eggs in mice, and staining and observation of cells and tissues. Our program has been well accepted because it emphasizes hands-on activities for each participant, and we have devised textbooks, DVDs, and prepared slides that can be used as home study materials.

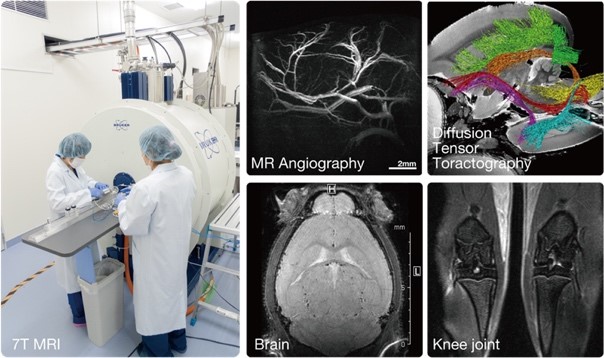
In in vivo experimental medicine, our ultimate goal is to elucidate human biological functions and conquer diseases. To this end, CIEA has spent many years developing its image analysis infrastructure technology, and in 2017, it established a new Live Animal Imaging Center. For more than 10 years since the days of our forerunner, the Image Analysis Laboratory, we have consistently developed the infrastructure for in vivo imaging using magnetic resonance imaging (MRI) and micro-X-ray computed tomography. The greatest advantage of in vivo imaging is its ability to observe a single animal noninvasively and over time. This is a potent approach that can contribute to laboratory animal science by helping to address one of the most important issues in animal experimentation today, the 3Rs of Replacement, Reduction, and Refinement of experimental animals. Furthermore, in vivo imaging can be used to observe dynamic pathological changes in animal models of various diseases and to accelerate proof-of-concept (POC) studies for pre- and post-treatment evaluation of diseases and translational research that leads to clinical trials in humans.
On January 10, 2020, CIEA was awarded the Minister of State for Health and Medical Care Strategies
Prize at the 3rd Japan Medical Research and Development Awards, for its “contribution to R&D in the
medical field through the development of cutting-edge laboratory animals.” The Awards are presented to
honor achievements that have made a significant contribution to the promotion of R&D in the medical
field, thereby deepening public interest and understanding and increasing incentives for researchers
and others. The Minister of State for Health and Medical Care Strategies Prize is given to a project
that has made a particularly significant contribution.
Since its establishment in 1952, CIEA has contributed substantially to the development of laboratory
animal science in Japan by establishing techniques for breeding laboratory animals and conducting
research on animal quality control. In addition, CIEA has established the scientific field of in vivo
experimental medicine by developing and providing animal testing systems for translational research
linking clinical and basic research. We believe that we were given this award for our contribution to
the development of medicine through laboratory animal science, encompassing our activities to date
since our founding.

The rasH2 mouse is a transgenic animal carrying the human oncogene (c-Ha-ras) and was developed in 1990 in a joint research project between CIEA, Tokai University, and the National Cancer Center. In 1993, a practical application study for carcinogenicity testing was initiated in collaboration with Keio University and the National Institute of Health Sciences. First, a system for mass production of rasH2 mice was established, and then the superiority of rasH2 over conventional animals was examined to determine whether it could be used in carcinogenicity tests. The results indicated that the originally anticipated test period could be significantly shortened to approximately six months for rasH2 mice compared to the two years required by conventional animals. In addition, validation studies were conducted over a five-year period from 1997 to 2001 under the leadership of the International Life Science Institute (ILSI) and the Health and Environmental Science Institute (HESI), in collaboration with more than 50 industry, government, and academic institutions in Japan, the U.S., and the EU; the rasH2 mouse was found to be more useful than other evaluation models. Since then, as more and more experience has been accumulated, especially by U.S. pharmaceutical companies, discussions have begun to explore the possibility of using this mouse study effectively as a new method for evaluating the carcinogenicity of drugs based on a “weight-of-evidence approach.” In 2021, the rasH2 mouse was listed as the global standard animal for oncogenicity testing using genetically modified mice in the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use S1B Addendum to the ICH guidelines (ICH S1B(R1)), with an individual name and specific usage.
PAGE TOP