Reproductive Engineering Laboratory carries out research and development of technologies, instruments, and resources related to reproductive engineering.
Major purposes include the collection, cryopreservation, and restoration of "original species" as the basis of the quality standards for laboratory animals, from creation to supply of the materials used in experiments, and the creation of new laboratory animals.
CIEM has been developing and improving reproductive engineering technologies for 40 years. For example, the cryopreservation technique of embryos was put into practical use in the 1980s for mice and in the 1990s for rats, and the Resource Bank was established in 1995 for strain preservation. Creation of genetically modified animals started in the 1980s for mice and in the 1990s for rats.
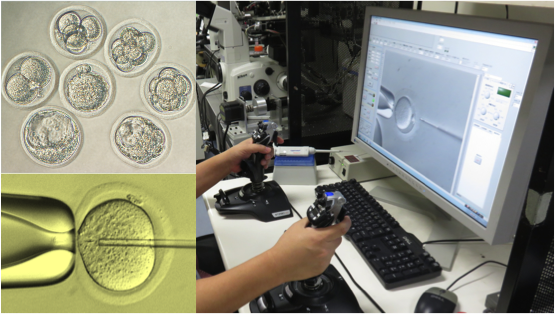
Nowadays, we focus on research related to cryopreservation of germ cells, cultured cells, tissues, and micromanipulation for the creation of genetically modified animals. For example, a recently developed vitrification method and solution for embryos (of rats, mice, etc.) and ES cells (in primates, etc.) is generally used with the commercialized vitrification solutions P10 and PEPeS. In the research and development for automated micromanipulation, the Integrated Automatic Embryonic Manipulation System (IAEMS*) has been established using an automated and motorized manipulator and reproducible results have already been confirmed in DNA injection and ES cell injection in mice, as well as in intracytoplasmic sperm injection (ICSI) in rats and mice. As reproductive engineering is necessary for fields such as laboratory animal science, medical science, pharmaceutical science, and biology, we will continue with our research and development.
*Integrated Automatic Embryonic Manipulation System
PAGE TOP