Liver Engineering Laboratory
Liver Engineering Laboratory has been creating a new humanized animal model by modification of NOG mice, especially focusing on development of a liver-humanized model.
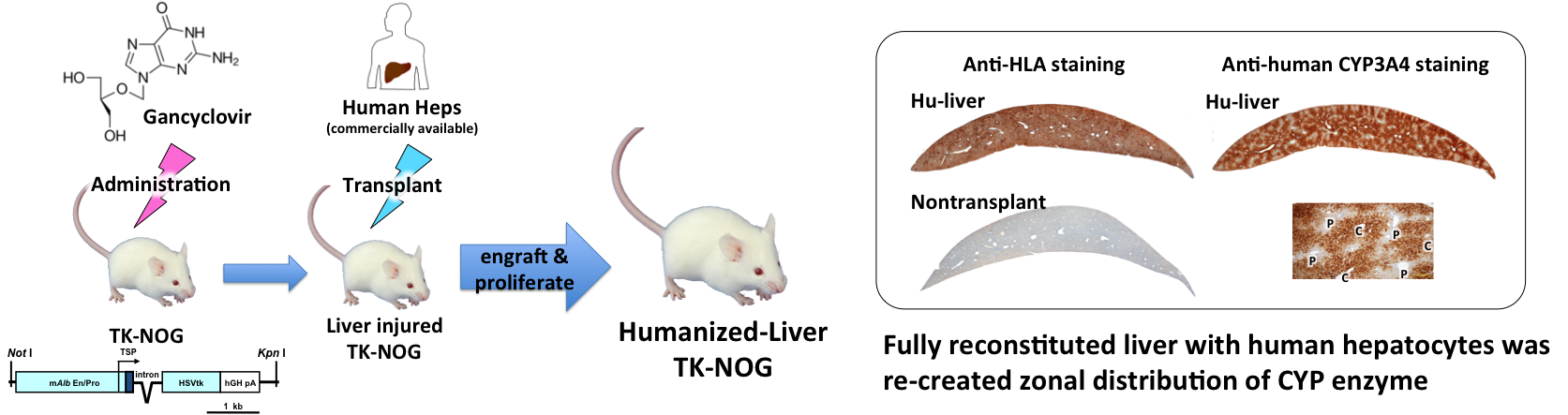
We established TK-NOG strain which is a drug-induced liver failure model.
The expression unit of herpes simplex virus thymidine kinase was introduced to NOG mouse by using microinjection technique.
The TK-NOG mice can bear human hepatocytes at high rates on the basis of extremely high acceptability of xenogeneic cells.
We succeeded to replace the mouse liver nearly 80% with human hepatocytes.
The "humanized liver" reconstructed with human hepatocytes has been shown to have drug metabolizing properties similar to those of the human liver.
In addition, we further improve the NOG mouse by using a microsatellite marker assisted selection protocol (known as speed congenic)
to efficiently replace the genetic background of conventional transgenic mouse and knockout mouse to that of NOG strain (super immunnodeficient) while preserving the characteristics.
※Reference
-
Drug transporter expression and activity in cryopreserved human hepatocytes isolated from chimeric TK-NOG mice with humanized livers.
Zerdoug A et al. Toxicol In Vitro. 2023 Aug;90:105592.
-
HepaSH cells: Experimental human hepatocytes with lesser inter-individual variation and more sustainable availability than primary human hepatocytes.
Uehara S et al. Biochem Biophys Res Commun. 2023 Jun 30;663:132-141.
-
Roles of human cytochrome P450 3A4/5 in dexamethasone 6beta-hydroxylation mediated by liver microsomes and humanized liver in chimeric mice metabolically suppressed with azamulin.
Uehara S et al. Drug Metab Pharmacokinet. 2023 Jun;50:100504.
-
o-Toluidine metabolism and effects in the urinary bladder of humanized-liver mice.
Yokota Y et al. Toxicology. 2023 Apr;488:153483.
-
The Unique Human N10-Glucuronidated Metabolite Formation from Olanzapine in Chimeric NOG-TKm30 Mice with Humanized Livers.
Uehara S et al. Drug Metab Dispos. 2023 Apr;51(4)480-491.
-
Human hepatocyte PNPLA3-148M exacerbates rapid non-alcoholic fatty liver diseas development in chimeric mice.
Kabbani M et al. Cell Rep. 2022 Sep 13;40(11):111321.
-
Humanized liver TK-NOG mice with functional deletion of hepatic murine cytochrome P450s as a model for studying human drug metabolism.
Uehara S et al. Sci Rep. 2022 Sep 1;12(1):14907.
-
Inhibition of nonhomologous end joining-mediated DNA repair enhances anti-HBV CRISPR therapy.
Murai K et al. Hepatol Commun. 2022 Sep;6(9):2474-2487.
-
Cytochrome P450-dependent drug oxidation activities and their expression levels in liver microsomes of chimeric TK-NOG mice with humanized livers.
Uehara S et al. Drug Metab Pharmacokinet. 2022 Jun;44:100454.
-
Probe drug T-1032 N-oxygenation mediated by cytochrome P450 3A5 in human hepatocytes in vitro and in humanized-liver mice in vivo.
Uehara S et al. Drug Metab Pharmacokinet. 2022 Jun;44:100453.
-
Comparison of mouse and human cytochrome P450 mediated-drug metabolising activities in hepatic and extrahepatic microsomes.
Uehara S et al. Xenobiotica. 2022 Mar;52(3):229-239.
-
An improved TK-NOG mouse as a novel platform for humanized liver that overcomes limitations in both male and female animals.
Uehara S et al. Drug Metab Pharmacokinet. 2022 Feb;42:100410.
-
Capsid Allosteric Modulators Enhance the Innate Immune Response in Hepatitis B Virus-Infected Hepatocytes During Interferon Administration.
Fukutomi K et al. Hepatol Commun. 2022 Feb;6(2):281-296.
-
Forward and reverse dosimetry for aniline and 2,6-dimethylaniline in humans extrapolated from humanized-liver mouse data using simplified physiologically based pharmacokinetic models.
Miura T et al. J Toxicol Sci. 2022;47(12):531-538.
-
Oxidative metabolism and pharmacokinetics of the EGFR inhibitor BIBX1382 in chimeric NOG-TKm30 mice transplanted with human hepatocytes.
Uehara S et al. Drug Metab Pharmacokinet. 2021 Dec;41:100419.
-
UDP-glucuronosyltransferase 1A4-mediated N2-glucuronidation is the major metabolic pathway of lamotrigine in chimeric NOG-TKm30 mice with humanised-livers.
Uehara S et al. Xenobiotica. 2021 Oct;51(10):1146-1154.
-
Hepatotoxicological potential of P-toluic acid in humanised-liver mice investigated using simplified physiologically based pharmacokinetic models.
Miura T et al. Xenobiotica. 2021 Jun;51(6):636-642.
-
Methyl-hydroxylation and subsequent oxidation to produce carboxylic acid is the major metabolic pathway of tolbutamide in chimeric TK-NOG mice transplanted with human hepatocytes.
Uehara S et al. Xenobiotica. 2021 May;51(5):582-589.
-
Plasma and hepatic concentrations of acetaminophen and its primary conjugates after oral administrations determined in experimental animals and humans and extrapolated by pharmacokinetic modeling.
Toda A et al. Xenobiotica. 2021 Mar;51(3):316-323.
-
In vivo drug interactions of itopride and trimethylamine mediated by flavin-containing monooxygenase 3 in humanized-liver mice.
Shimizu M et al. Drug Metab Pharmacokinet. 2021 Apr;37:100369.
-
Metabolic Profiles of Tetrabromobisphenol A in Humans Extrapolated from Humanized-Liver Mouse Data Using a Simplified Physiologically Based Pharmacokinetic Model.
Miura T et al. Chem Res Toxicol. 2021 Feb 15;34(2):522-528.
-
Identification of human long noncoding RNAs associated with nonalcoholic fatty liver disease and metabolic homeostasis.
Ruan X et al. J Clin Invest. 2021 Jan 4;131(1):e136336.
-
Pharmacokinetics of primary oxidative metabolites of thalidomide in rats and in chimeric mice humanized with different human hepatocytes.
Miura T et al. J Toxicol Sci. 2021;46(7):311-317.
-
Roles of human cytochrome P450 1A2 in coumarin 3,4-epoxidation mediated by untreated hepatocytes and by those metabolically inactivated with furafylline in previously transplanted chimeric mice.
Miura T et al. J Toxicol Sci. 2021;46(11):525-530.
-
Different Hepatic Concentrations of Bromobenzene, 1,2-Dibromobenzene, and 1,4-Dibromobenzene in Humanized-Liver Mice Predicted Using Simplified Physiologically Based Pharmacokinetic Models as Putative Markers of Toxicological Potential.
Miura T et al. Chem Res Toxicol. 2020 Dec 21;33(12):3048-3053.
-
Human plasma concentration-time profiles of troglitazone and troglitazone sulfate simulated by in vivo experiments with chimeric mice with humanized livers and semi-physiological pharmacokinetic modeling.
Ito S et al. Drug Metab Pharmacokinet. 2020 Dec;35(6):505-514.
-
Comparative Transcriptomics Analyses in Livers of Mice, Humans, and Humanized Mice Define Human-Specific Gene Networks.
Jiang C et al. Cells. 2020 Nov 30;9(12):2566.
-
Predicted values for human total clearance of a variety of typical compounds with differently humanized-liver mouse plasma data.
Nakayama K et al. Drug Metab Pharmacokinet. 2020 Aug;35(4):389-396.
-
Prediction of circulating human metabolites of pemafibrate, a novel antidyslipidemic drug, using chimeric mice with humanized liver.
Ogawa SI et al. Xenobiotica. 2020 Jul;50(7):769-775.
-
Human Aldehyde Oxidase 1-Mediated Carbazeran Oxidation in Chimeric TK-NOG Mice Transplanted with Human Hepatocytes.
Uehara S et al. Drug Metab Dispos. 2020 Jul;48(7):580-586.
-
A novel Css-MRTpo approach to simulate oral plasma concentration-time profiles of the partial glucokinase activator PF-04937319 and its disproportionate N-demethylated metabolite in humans using chimeric mice with humanized livers.
Kamimura H et al. Xenobiotica. 2020 Jul;50(7):761-768.
-
Metabolism of desloratadine by chimeric TK-NOG mice transplanted with human hepatocytes.
Uehara S et al. Xenobiotica. 2020 Jun;50(6):733-740.
-
Different Roles of Human Cytochrome P450 2C9 and 3A Enzymes in Diclofenac 4'- and 5-Hydroxylations Mediated by Metabolically Inactivated Human Hepatocytes in Previously Transplanted Chimeric Mice.
Miura T et al. Chem Res Toxicol. 2020 Feb 17;33(2):634-639.
-
Hepatitis C virus infection suppresses hepatitis B virus replication via the RIG-I-like helicase pathway.
Murai K et al. Sci Rep. 2020 Jan 22;10(1):941.
-
Expansion, in vivo-ex vivo cycling, and genetic manipulation of primary human hepatocytes.
Michailidis E et al. Proc Natl Acad Sci U S A. 2020 Jan 21;117(3):1678-1688.
-
In vivo functional analysis of non-conserved human lncRNAs associated with cardiometabolic traits.
Ruan X et al. Nat Commun. 2020 Jan 2;11(1):45.
-
Expression and inducibility of cytochrome P450s in human hepatocytes isolated from chimeric mice with humanised livers.
Uehara S et al. Xenobiotica. 2019 Jun;49(6):678-687.
-
Chimeric mice with humanized liver as a model for testing organophosphate and carbamate pesticide exposure.
Suemizu H et al. Pest Manag Sci. 2018 Jun;74(6):1424-1430.
-
Sodium taurocholate cotransporting polypeptide inhibition efficiently blocks hepatitis B virus spread in mice with a humanized liver.
Nakabori T et al. Sci Rep. 2016 Jun 9;6:27782.
-
The human hepatic cell line HepaRG as a possible cell source for the generation of humanized liver TK-NOG mice.
Higuchi Y et al. Xenobiotica. 2014 Jan;44(2):146-53.
-
The reconstituted 'humanized liver' in TK-NOG mice is mature and functional.
Hasegawa M et al. Biochem Biophys Res Commun. 2011 Feb 18;405(3):405-10.
